Antibiotic combinations reduce Staphylococcus aureus clearance - Nature.com
Abstract
The spread of antibiotic resistance is attracting increased attention to combination-based treatments. Although drug combinations have been studied extensively for their effects on bacterial growth1,2,3,4,5,6,7,8,9,10,11, much less is known about their effects on bacterial long-term clearance, especially at cidal, clinically relevant concentrations12,13,14. Here, using en masse microplating and automated image analysis, we systematically quantify Staphylococcus aureus survival during prolonged exposure to pairwise and higher-order cidal drug combinations. By quantifying growth inhibition, early killing and longer-term population clearance by all pairs of 14 antibiotics, we find that clearance interactions are qualitatively different, often showing reciprocal suppression whereby the efficacy of the drug mixture is weaker than any of the individual drugs alone. Furthermore, in contrast to growth inhibition6,7,8,9,10 and early killing, clearance efficacy decreases rather than increases as more drugs are added. However, specific drugs targeting non-growing persisters15,16,17 circumvent these suppressive effects. Competition experiments show that reciprocal suppressive drug combinations select against resistance to any of the individual drugs, even counteracting methicillin-resistant Staphylococcus aureus both in vitro and in a Galleria mellonella larva model. As a consequence, adding a β-lactamase inhibitor that is commonly used to potentiate treatment against β-lactam-resistant strains can reduce rather than increase treatment efficacy. Together, these results underscore the importance of systematic mapping the long-term clearance efficacy of drug combinations for designing more-effective, resistance-proof multidrug regimes.
Main
Antibiotics are the most effective tool to treat bacterial infections, but bacteria are rapidly developing both resistance and persistence to these drugs18,19. The use of antibiotics has increased steadily over the past 15 years and is now further increasing due to the ongoing coronavirus pandemic; nearly all patients with severe COVID-19 are receiving antibiotics to prevent and treat secondary bacterial infections20. Meanwhile, bacterial pathogens—most prominently, the widespread opportunistic pathogen Staphylococcus aureus—are evolving ways to evade antibiotic killing through various mechanisms of resistance21,22 as well as persistence23,24, whereby a small isogenic subpopulation of bacteria undergoes a phenotypic change to temporarily avoid the cidal effect of the antibiotic25. Antibiotic persistence leads to a biphasic killing curve, beginning with an early phase of rapid killing of the normal cells and continuing with a later phase of a much slower killing of the persistent cells26. Persistence can therefore reduce the ultimate infection clearance efficacy of antibiotic treatments27.
As pathogens evolve resistance and persistence to antibiotics, drug combinations are gaining increased attention28. Multidrug therapies can have potential benefits in increasing the spectrum of targeted pathogens, preventing the emergence of antibiotic resistance and improving clinical efficacy29,30,31,32,33. Considering the effects of subinhibitory drug combination on bacterial growth (growth–inhibition interactions), laboratory studies have found that the potency of drug mixes increases with the number of co-mixed drugs, yet specific drug pairs and multidrug combinations can substantially deviate from this general trend due to drug synergy and antagonism1,2,3,4,5,6,7,8,9,10,11. Considering the bactericidal effects of drug combination, drug antagonism is commonly revealed, especially between static and cidal drugs2,3,34,35,36,37,38,39,40. In some cases, drug antagonism can be so strong that the combined effect of co-mixed drugs is weaker not only compared with their expected additive effects but also compared with the effect of one of the drugs alone12,31,32,33,41. Such suppressive, or hyperantagonistic, interactions are typically non-reciprocal—the combined effect of the drugs is weaker than one of the mixed components but not the other (one drug suppresses the effect of the other, but not vice versa)2,12,33,41. In principle, drug combinations of which the effect is weaker than the effects of either one of the individual drug components are also possible (reciprocal suppression). However, despite the potential clinical implications of such reciprocal suppression—which, while jeopardizing drug efficacy, may counteract resistance12,31,32,33—such extreme interactions are not observed in drug combination effects on bacterial growth inhibition or on the early-killing phase.
Although drug combination effects on growth inhibition have been systematically mapped1,2,3,4,5,6,7,8,9,10,11, much less is known about how drugs combine to affect longer-term population clearance and persistence (clearance interactions)12,13,14. Measuring bacterial survival after prolonged exposures to individual drugs and drug combinations is inherently difficult as it requires the quantification of low densities of surviving cells, and the use of conventional plating techniques limit the number of antimicrobial combinations that can be tested42. Initial studies mapping the effect of specific drug pairs on intermediate (2–4 h) or long-term clearance (8 h) identified synergistic, antagonistic as well as non-reciprocal suppressive interactions12,13,14. At longer-term survival (1 day exposure), there is even an example of reciprocal suppression43. Owing to the lack of a high-throughput approach to quantify cell viability en masse, a systematic study of such clearance interactions is lacking. It is therefore unclear to what extent these clearance interactions correlate with early-killing and growth-inhibition interactions, how the overall clearance efficacy varies with the number of co-mixed drugs, and whether reciprocal suppression could be identified and used to counteract resistance to the single drugs.
High-throughput cell-viability assay
To systematically quantify bacterial survival under a range of drug combinations, we adapted a high-throughput cell-viability assay based on miniaturized plating and automated image analysis44. In brief, growing S. aureus bacterial cultures were treated with individual drugs or drug mixes. Then, at several time points after drug exposure, the cultures were microplated at a range of dilutions. Automated imaging and image analysis enabled the quantification of the number of microcolonies grown at each microplating spot (up to 100 colonies were detected in each microplating spot in a 96-well format) (Methods and Fig. 1a). Detection sensitivity and reliable colony counting was facilitated by differential labelling and co-culturing of the same S. aureus strain with two different fluorescent markers (DsRed and GFP). Using this assay, we comprehensively quantified the effect of pairwise and multidrug mixes on the early- and late-killing phases of a drug-sensitive S. aureus strain as well as on the competition among drug-sensitive and drug-resistant strains (Methods; a total of over 25,000 microplating images).
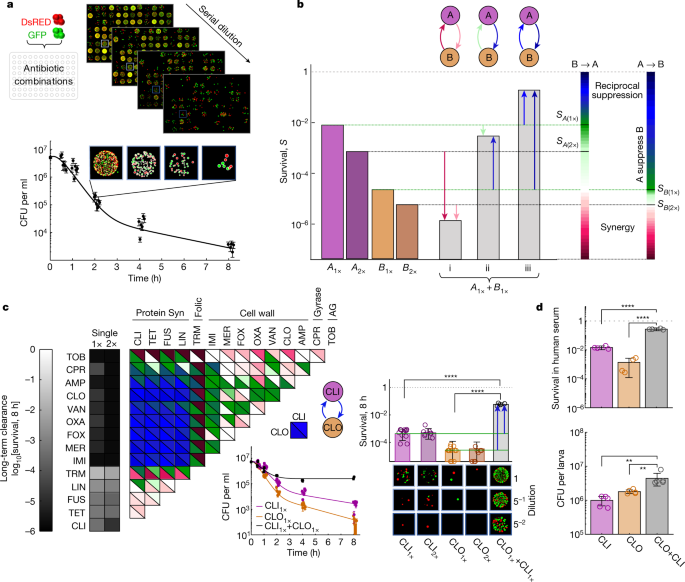
a, Cell viability over time after antibiotic treatment was measured by high-throughput microplating and automated image analysis of a mixed DsRed- and GFP-tagged S. aureus strain. Example data are shown for treatment with CLI (n = 6 wells; fivefold serial dilution microplate images are shown for one replicate at one time point). b, Schematic of directional interactions. Contrasting survival after a combined treatment with two drugs, A and B, at a fixed cidal concentration (Supplementary Table 1, grey bars, SA+B); the survival under drugs A and B alone at the same or double the concentration (SA(1×), SA(2×) (purple bars); SB(1×), SB(2×) (orange bars)) defines two-directional interaction scores for the effect of drug B on drug A (B→A, left colour scale) and the effect of A on B (A→B, right colour scale). The combined effect could be synergistic (SA+B < min[SA(2×), SB(2×)]) (i); non-reciprocal suppressive (SB(1×) < SA+B < SA(1×), A suppresses B) (ii); or reciprocal suppressive (SA+B > max[SA(1×), SB(1×)]) (iii). c, Measurements of directional interaction scores for pairwise combinations of 14 antibiotics in long-term clearance efficacy (2 strains were measured in A1×, A2× and A1× + B1× in 6, 4 and 2 experiments, respectively). The top (bottom) triangles show the interaction score for the effect of the drugs in the row (column) on the drugs in the column (row). Inset: the killing curves and final survival fraction for a representative suppressive drug combination (CLI + CLO). Statistical analysis was performed using z-tests; P < 10−10. Points on the axis are below the detection limit. For each treatment, representative microplating images of one replicate are shown. d, Bacteria viability after 8 h exposure to CLI–CLO in human serum (top; 2 strains were measured in 2 experiments; P < 10−10, two-tailed z-tests) and in G. mellonella larvae (d, bottom; n = 10 AROLarvae, homogenized and plated in 5 pairs; from left to right, P = 0.008 and P = 0.008, two-tailed Mann–Whitney U-tests). For a, c and d, the error bars represent the 95% confidence intervals calculated from the colony counts by the Poisson's model (a and c (middle)) or 2× s.e.m. (c (right) and d).
Source data
Drug interactions in early killing
Mapping the growth inhibition and early-killing interactions among all pairwise combinations within a set of 14 diverse antibiotics (Supplementary Table 1) revealed two distinct networks of synergistic, antagonistic and non-reciprocal suppressive interactions (Extended Data Fig. 1). Inspired by previous studies7,12, we defined for each pair of drugs A and B two-directional interaction scores signifying the effect of drug A on drug B and the effect of drug B on drug A. For early killing, directional interactions were defined on the basis of measurements of the decrease in cell viability during 90 min after exposure to each of the 14 drugs when applied individually (each drug A is applied at a fixed cidal concentration A1× chosen on the plateau of its dose–response curve, and at double this concentration, A2× = 2 × A1×; Extended Data Fig. 2a and Supplementary Table 1) and when applied in pairwise combinations (A1× + B1×) (Methods and Fig. 1b). For growth inhibition, directional interactions were defined based on measurements of the 90% inhibition isobole in two-dimensional concentration gradients of each drug pair (Methods and Extended Data Figs. 1a–d and 3). Both the early-killing and growth-inhibition networks showed interactions ranging from synergistic to antagonistic and non-reciprocal suppression, yet these interactions were not correlated among the two networks (Extended Data Fig. 2b) (for example, protein-synthesis inhibitors suppress the efficacy of trimethoprim on the growth inhibition but not on the killing efficacy) (Extended Data Figs. 1e,f and 3–5). Non-reciprocal suppression was more common in killing interactions, mirroring the classical suppression of the killing activity of cidal drugs by static protein synthesis drugs2,3,38,45,46 (even though these 'classically static' drugs are in fact bactericidal against S. aureus at concentrations used clinically and as used in our study47,48,49). Although non-reciprocal suppression was common, reciprocal suppression was not detected in the inhibitory or the early-killing networks (Extended Data Figs. 1e,f, 3 and 4).
Drug interactions in long-term clearance
In contrast to early-killing and growth-inhibition interactions, quantifying the effects of antibiotic combinations on long-term clearance revealed pronounced and widespread reciprocal suppression, especially among combinations of cell-wall and classically static protein-synthesis inhibitors (Fig. 1c). To quantify clearance interactions, we measured cell viability during 8 h exposure to cidal concentrations of individual drugs and drug pair combinations and calculated their directional interaction scores (as defined above) (Fig. 1b and Methods). These clearance interactions were not correlated with the growth inhibition interactions but were strongly correlated with the early-killing interactions, indicating that the long-term effect of the drug combinations can be predicted, to some extent, from the early-killing efficacy (Extended Data Fig. 2c,d). However, we also discovered some substantial differences between the early-killing and late-clearance interactions. Indeed, some strong non-reciprocal suppressive early-killing interactions, such as the suppression of the early-killing activity of the cidal drugs by the classically static drugs, are weakened or completely diminished at longer time scales (such as the effect of protein-synthesis inhibitors on the early-killing efficacy of tobramycin and the effect of the trimethoprim on the early-killing efficacy of cell-wall inhibitors or ciprofloxacin) (Fig. 1c and Extended Data Figs. 1f, 4 and 5). By contrast, many other known early-killing non-reciprocal suppressive interactions were strongly enhanced at longer time scales, often developing into reciprocal suppression. In particular, we found that, when cell-wall and classically static protein-synthesis inhibitors are combined, the persisting bacterial population is larger than when any of the drugs are administered alone, even at the same dose and despite using both drugs at cidal concentrations (Fig. 1c and Extended Data Fig. 5). These reciprocal suppressive clearance interactions were robust to changes in drug concentrations, growth environments, incubation time and bacterial physiological state, and were also present in an unrelated S. aureus strain with a different genetic background (Methods and Extended Data Fig. 6a–e). Furthermore, such suppression also appeared in human serum and in an in vivo infection model of the larva of the great wax moth (G. mellonella) (Methods, Fig. 1d and Extended Data Fig. 6b,d,f,g). However, note that suppression observed in the larvae is weaker, especially in the direction of protein-synthesis inhibitors suppressing β-lactams and seems dependent on the physiological stage of the larvae, as well as on drug concentrations (in particular, suppression was not observed at lower drug concentrations and in competition among strains) (Extended Data Fig. 6h,i).
Multidrug-reduced long-term clearance
Not only was reciprocal suppression common among drug pairs, increasing the number of drugs beyond two further reduced the efficacy of long-term clearance. Following the same procedure used for the drug pairs, we measured the population survival in the presence of all possible multidrug mixes of six commonly used cidal and classically static drugs (clindamycin (CLI), tetracycline (TET), fusidic acid (FUS), meropenem (MER), ciprofloxacin (CPR), oxacillin (OXA); 63 combinations) (Fig. 2a, Extended Data Fig. 7a and Supplementary Table 1). In contrast to the growth inhibitory effects of multidrug combinations, for which potency increases with the number of drugs6,7,8,9,10, focusing on long-term clearance, we found the opposite behaviour—increasing the number of drugs reduces the overall clearance efficacy of the combinations (Fig. 2a and Extended Data Fig. 7a,b). This phenomenon is unique to long-term clearance interactions; whereas long-term efficacy decreased with the number of drugs, early-killing efficacy did not change across the different multidrug combinations (Extended Data Fig. 7c). The same trend was also observed in an independent set of five additional drugs of diverse classes (cefoxitin (FOX), linezolid (LIN), cefazolin (CEF), minocycline (MIN) and pristinamycin (PRI); Fig. 2b and Extended Data Fig. 8). Furthermore, considering each multidrug set as a combination of two drug mixes (for example, mix ABC is decomposed to AB + C, A + BC, AC + B), we found enrichment of reciprocal suppressions between drug mixes (Fig. 2c and Extended Data Fig. 7d), which was not observed in early-killing interactions (Extended Data Fig. 7e). Separating drug sets by all-cidal and all-static or combined drug mixes highlights that this reciprocal suppression is mostly driven by combinations of cidal and classically static drugs (Fig. 2b,c). However, including specific drugs of which the cidal activity is only weakly dependent on cell metabolism15,16,17 strongly synergized the clearance efficacy of all of the other drugs and drug mixes, efficiently eradicating the long-term surviving cell population (persistence-targeting: daptomycin (DAP), mitomycin (MIT)) (Fig. 2b,d and Extended Data Fig. 8). Our results thereby indicate that, in contrast to their combined effect on growth inhibition, multidrug mixtures commonly reduce rather than increase long-term clearance efficacy and that, although common among many drug classes, such reduced efficacy can be prevented by drugs that are effective against metabolically inactive cells.
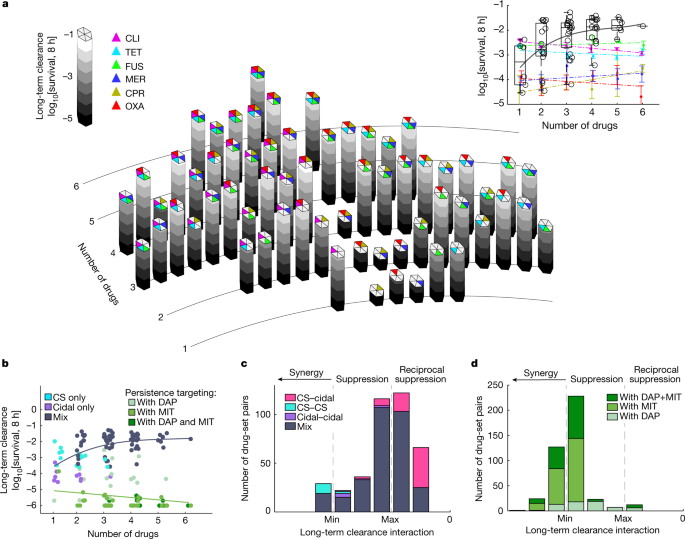
a, Bacterial survival fraction after 8 h exposure to all possible combinations of six drugs (26 = 64), including 3 commonly used cidal (MD-cidal: MER, CPR, OXA) and 3 classically static drugs (CS: CLI, TET, FUS; Supplementary Table 1). Two strains were measured in two experiments. Inset: the survival fraction as a function of the number of co-mixed drugs (black circles; in contrast to survival fraction under each of the single drugs at increased dosage from 1× to 6× of the dosage used in the combination (coloured dots, coloured linear fits; data are mean ± 95% confidence intervals). The box plots show the median (centre line); the upper and lower quartiles (box limits); and the maximum and minimum values (whiskers); the grey line shows the quadratic fit. b, The survival fraction as described in the inset of a, but including combinations of two additional cidal (FOX, CEF) and three additional CS drugs (LIN, MIN, PRI) as well two weakly metabolic-dependent cidal drugs (MIT, DAP; persistence-targeting) (Extended Data Fig. 8). The dot colour represents drug combinations that are only CS (cyan), only cidal (purple) or a mix (grey, black cubic fit), and sets including persistence-targeting drugs (green shades; green linear fit). c,d, The distribution of the interaction scores among all tested non-overlapping pairs of drug sets (for example, drug mix ABC is decomposed into AB + C, A + BC, AC + B) without (c) and with (d) persistence-targeting drugs. n = 391 (c) and n = 422 (d). The combined effect of the drug mix is shown relative to the minimum (min) and maximum (max) of the effect of the two drug sets (Methods). Drug mix pairs are classified as only cidal (purple), only CS (cyan), mix of all CS and all cidal (pink), pairs of mixed drug sets (mix, grey) or sets including DAP or MIT (green shades).
Source data
Selection against antibiotic resistance
Next, we competed drug-resistant and drug-sensitive strains in mixtures of reciprocal suppressive drug pairs, and we found that these interactions can select against strains that are resistant to either of the single drugs. It has been shown that non-reciprocal suppressive interactions can reverse the selective advantage of a strain that is resistant to one of the drugs (if drug A suppresses drug B, the combination of the two drugs selects against resistance to drug A, but not against resistance to drug B)12,33. Extrapolating from these results, it has been hypothesized that, with reciprocal suppression, it might be possible to select against any of the single-drug-resistant mutants31. Focusing on the combination of TET and ampicillin (AMP) (a reciprocal suppressive pair for long-term clearance; Fig. 1c), we competed our sensitive ancestral strain with either a strain that is resistant to TET (TET evolved) (Methods, Fig 3a and Extended Data Fig. 9c,d) or a strain that is resistant to cell-wall inhibitors (methicillin-resistant Staphylococcus aureus (MRSA) (Fig. 3b and Extended Data Fig. 9e–g), or we compared the sensitive strain with and without an inducible β-lactamase (Methods and Extended Data Fig. 9a,b)). By contrasting the survival of the drug-sensitive and drug-resistant strains after 8 h of exposure to a two-dimensional concentration gradient of TET and AMP, we found that, under an extended region of the two-drug concentration space, the long-term survival of the resistant strains was much lower compared with the sensitive strain (10-fold to 100-fold) (Extended Data Fig. 9a–g). These selection inversions resulted from the interaction itself; there was no selection against the resistant strains under any of the single treatments. Selection against each of the single-drug-resistant mutants also occurred in competition within human serum (Fig. 3c,e and Extended Data Fig. 9h) and selection against MRSA was even observed within the G. mellonella infection model (in TET–AMP (Fig. 3f) as well as in MIN–AMP (Methods and Extended Data Fig. 9i). However, note that in this in vivo environment, even the single drugs, TET and MIN, showed selection against the MRSA strain (Fig. 3f and Extended Data Fig. 9i; perhaps reflecting suppressive interactions of these drugs with natural antimicrobial peptides in vivo)50. Selection against TET-resistant mutants did not occur within larvae, possibly due to the effect of oxygen depletion on the action of β-lactams51 and due to competition for resources within larvae (Fig. 3d).
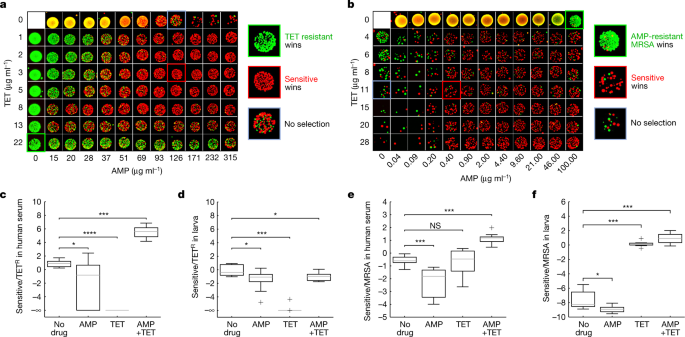
a,b, Microplating images of surviving colonies after 8 h competition of equally mixed sensitive (ATCC 29213) versus evolved TET-resistant strains (a; Sensitive, red; TET resistant, green) and sensitive versus MRSA strains (b; sensitive (red); AMP-resistant MRSA (green)) in a two-dimensional gradient of TET and AMP. Insets: magnified images under the selective drug alone (green; TET (a); AMP (b)), the non-selective drug (blue; AMP (a); TET (b)) and the combination (red; selecting against TETR (a); selecting against MRSA (b)). c,e, The log2-transformed ratio of the survival of the sensitive (green) and TET-resistant (c; TETR, red) or MRSA (e; red) strains after competing them in human serum with TET (2 μg ml−1 (c); 50 μg ml−1 (e)), AMP (150 μg ml−1 (c); 35 μg ml−1 (e)) and with TET + AMP for 8 h. Two strains measured in five experiments per condition. Statistical analysis was performed using two-sided Mann–Whitney U-tests; from left to right, P = 0.0207, P = 0.0001 and P = 0.0002 (c); P = 0.0006, P = 0.9097 and P = 0.0002 (e). d,f, The log2-transformed ratio of the survival of the sensitive and TET-resistant or MRSA strains measured 8 h after inoculation into larvae treated with TET (1.5 μg ml−1 (d); 50 μg ml−1 (f)), AMP (38 μg ml−1 (d); 100 μg ml−1 (f)) or TET–AMP. For d, n = 10 TrueLarvae per condition. For f, n = 8 AROLarvae per condition. For d and f, statistical analysis was performed using two-sided Mann–Whitney tests; from left to right, P = 0.0173, P = 0.0001 and P = 0.0211 (d); P = 0.0207, P = 0.0002 and P = 0.0002 (f). The box plots show the median (centre line); the upper and lower quartiles (box limits); and the maximum and minimum values (whiskers).
Source data
Reverse effect of resistance inhibition
Finally, given the ability of suppressive drug pairs to select against AMP resistance, we examined the effect of supplementing these drug combinations with a β-lactamase inhibitor. β-Lactamase inhibitors are commonly used together with β-lactams to increase treatment efficacy against strains carrying the β-lactamase enzyme. We measured the long-term survival of a β-lactamase-mediated AMP-resistant strain (Methods) as a function of increasing doses of avibactam, a known β-lactamase inhibitor, in the presence of three different clearance-suppressive drug combinations (AMP combined with TET, CLI or FUS; Fig. 4). Consistent with these combinations selecting against AMP resistance, we found that adding avibactam to these clearance-suppressive combinations decreased rather than increased the long-term clearance efficacy against the β-lactam-resistant strain (Fig. 4 and Extended Data Fig. 10a–d). Thus, in the presence of clearance-suppressive drug combinations, a β-lactamase inhibitor jeopardizes, rather than facilitates, treatment efficacy.
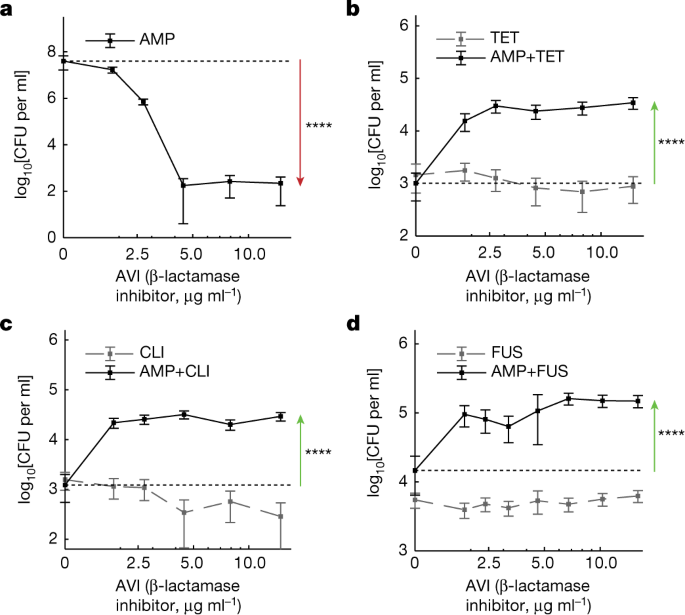
a, As expected, the killing efficacy of AMP at fixed dosage (0.5 μg ml−1) is enhanced by the addition of an increasing dosage of avibactam (AVI). b–d, By contrast, the treatment efficacy is reduced rather than enhanced when avibactam is added to a combination of AMP (0.5 μg ml−1) with a fixed dosage of TET (b; 20 μg ml−1), CLI (c; 10 μg ml−1) or AMP (d; 0.4 μg ml−1) with FUS (d, 20 μg ml−1). No AMP controls show that this inverted effect of AVI is mediated by the interaction among AMP and these protein-synthesis inhibitors (grey dashed lines). The error bars represent 95% confidence intervals calculated from the colony counts of one sample by the Poisson's model. Statistical analysis was performed using z-tests based on the Poisson-derived confidence intervals; P < 10−10.
Source data
Discussion
By systematically quantifying the growth inhibition and killing dynamic of individual drugs and drug combinations at cidal, clinically relevant concentrations, we found that reciprocal suppressive interactions emerge in prolonged treatment efficacy. In contrast to shorter-term drug effects and conventional wisdom, drug efficacy is reduced rather than increased as more drugs are combined. These strong suppressive clearance interactions are suggestive of induced persistence in the presence of specific drug combinations. Indeed, adding persistence-targeting, weakly metabolism-dependent drugs, such as mitomycin C and daptomycin, can completely restore the efficacy of these otherwise suppressive drug mixes, explaining the clinical success of drug combinations involving these drugs52. Clinicians should also be cautious in relying on growth inhibition phenotypes as a guide for prescribing drug combination therapies, as drug combinations with high inhibitory efficacy, such as TET and cell wall inhibitors53, may have reduced long-term efficacy. The lack of correlation between growth inhibition interactions and long-term clearance interactions is consistent with earlier observations14 and we hypothesize that it may stem from the differential dependencies of these interactions on the physiology of the population majority and population heterogeneity, respectively14,26. Furthermore, owing to these clearance-suppressive drug interactions, adding β-lactamase inhibitors can, counterintuitively, jeopardize the long-term clearance efficacy of the drug combinations against β-lactamase-carrying resistant strains, cautioning against certain clinically prescribed combinations such as the combination of macrolides or doxycycline with amoxicillin/clavulanate for the treatment of community-acquired pneumonia54,55. At the same time, reciprocal suppressive drugs also open up new opportunities for designing treatment regimens that are inherently selective against resistance to any one of their individual agents. However, note that further study will be needed to more fully understand the mechanism of drug-combination-induced bacterial persistence56,57, and the range of possible evolutionary paths that may enable bacteria to escape selection against resistance12. Although the results proved to be robust to changes in drug doses, external conditions and strain backgrounds, for assessing any clinical relevance, it will also be important to determine the extent to which they apply in other bacterial species, and in mammalian in vivo models. However, as drugs could interact in profoundly different ways in terms of their effect on growth and early and late population survival, our study underscores the importance of systematically quantifying the long-term clearance efficacy of drug combinations for guiding the design of multidrug treatments that may better prevent the evolution of drug resistance.
Methods
Strains and plasmids
The S. aureus sp. Rosenbach (ATCC 29213) strain was transformed with either a DsRed (pHC48, sarAP1_DsRed, CHLR) or a sGFP fluorescent reporter gene (pCM29, sarAP1_sGFP, CHLR) under a constitutive promoter58 (antibiotic-sensitive S. aureus). To test for generality and for counter-selection experiments, the MW2 S. aureus strain59 was similarly transformed with these two plasmids (MRSA) (Fig. 3b,e,f and Extended Data Fig. 9e–i). A β-lactamase-inducible plasmid was constructed by amplifying the blaZ gene from Enterococcus faecalis (ATCC 49757) and cloning by Gibson assembly under the TET-inducible promoter of the pG+off plasmid60. This plasmid was transformed into the sensitive S. aureus strain to form the AMPR strain in the presence of 0.05 μM anhydrotetracycline (Fig. 4 and Extended Data Figs. 9a,b and 10a–d). Laboratory evolution was performed according to a previously established protocol to evolve the antibiotic-sensitive S. aureus strain to TET61. A resistant clone with the highest TET minimal inhibitory concentration (25-fold increase compared with the ancestral strain) was transformed with either the DsRed or the GFP plasmid and used in the experiments of Fig. 3a,c,d and Extended Data Fig. 9c,d (TETR). Fresh antibiotic solutions were prepared from powder stocks (Sigma-Aldrich, Cayman chemical, AdooQ Bioscience) on a monthly basis, filter-sterilized and kept at −20 °C until use.
Quantifying bacterial survival under antibiotic exposure
We adapted a high-throughput cell-viability assay44 in which bacterial cultures were exposed to single or multiple drugs in 96-well assay plates and their viability across time was quantified by microplating and colony counting. Specifically, these experiments were performed in 6 steps. (1) Preparation of bacterial stocks. Single colonies of the GFP and DsRed fluorescently labelled sensitive S. aureus strains were isolated and grown overnight in LB supplemented with 10 μg ml−1 CHL (to maintain the plasmid) at 37 °C, 250 rpm. Overnight cultures were diluted 1:5,000 and grown to an optical density at 600 nm (OD600) of 0.25. These cultures were concentrated by centrifugation and small aliquots of 50 μl containing ~2.2 × 109 cells were frozen in glycerol (stored at −80 °C). (2) Preparing assay plates with antibiotics. Deep 96-well plates were filled with 600 μl LB and supplemented with single or multiple antibiotics using an automated digital dispenser (D300e, Tecan). At least two replicate wells were randomly distributed for each condition. (3) Antibiotic exposure. For each biological replicate, frozen cultures of the GFP and DsRed strains were thawed and mixed at a 1:1 ratio, diluted 1:250 in 25 ml LB and incubated for 40 min at 37 °C and 250 rpm. These co-growing bacterial cultures were then aliquoted into the 96-well deep-well antibiotic plates (150 μl culture; total volume of 750 μl) at a low inoculum density of about 106−107 CFU per ml to minimize the risk of pre-existing resistant mutants and prevent nutrient depletion (Extended Data Fig. 10j–m; the growth of the resistant strain was not limited in the supernatant of the FOX regimes that kill the sensitive strain (>1 μg ml−1) but not the resistant strain (<3.8 μg ml−1), especially in the wells in which the starting density of the sensitive strain was initially low (<107); this result indicates that nutrient depletion is negligible during our killing assay starting with a 107–106 initial population size). This inoculum density follows recommended clinical testing standards for killing assays42. No-cell and no-antibiotic wells were designated on each plate to control for contamination (2–5 of the wells in each plate). (4) Microplating assay for quantification of bacterial viability (colony-forming units (CFU)). At fixed time points after antibiotic exposure at 37 °C and 250 rpm (0, 0.25, 0.5, 1 and 1.5 h for the early-killing assay; and 0, 0.5, 1, 2, 4 and 8 h for the long-term clearance assay), small aliquots (10–150 μl) were taken from each well, and fivefold serial diluted for plating. The 8 h time point was the last time point for sampling as the clearance efficacy of many of the single drugs (Extended Data Fig. 5) already reached the detection limit of our assay (1 colony in 7 μl volume plated; ~140 CFU per ml; extending beyond 8 h requires larger plating volumes, which cannot be done in microplates). Large-volume experiments for the two chosen drug pairs showed robustness of the clearance interactions even at 24 h (Extended Data Fig. 6c). For the 8 h clearance assay, in which cell viability declined substantially, we also plated the undiluted culture (dilutions of 5n, where n = 0–4) and therefore added a wash step by centrifugation and resuspension to remove the drugs (three washes with 800 μl PBS at 4,000 rpm for 10 min). For the killing-assay, in which cell viability was higher, an initial dilution of at least 50× was used, precluding the washing step (final dilutions of 50 × 5n, where n = 0–2). Small microdrops (7 µl) from each dilution were then carefully microplated (Gilson 96-channel plate master) in one to four technical replicates onto omnitray single-well (Greiner) plates filled with 45 ml of agar with 0.2× diluted LB and incubated for 24 h at 37 °C. The diluted LB was used to limit the substrate availability on the agar thereby allowing the growth of many small colonies on a relatively small surface area62. (5) Imaging. After incubation, the agar plates were imaged for GFP (excitation:470/30; emission: 540/50) and DsRed (excitation: 590/30; emission 641/75) with a custom-made automated macroscope device63. (6) Detection of resistance. At the last time point, 15 µl aliquots from each well of the assay plates were also inoculated into a 96-well plate with 200 µl liquid LB containing, in each well, the same antibiotic concentrations of the assay plates and incubated overnight at 37 °C at 250 rpm. After incubation, the growing cultures (OD600 > 0.2), indicating resistant cells, were excluded from further analysis. Overall, our data included ~15,000 microdrops: n = 2 biologically independent strains (DsRed, GFP) measured in 585 drug conditions (28 singles (1×, 2× dose) and 91 pairs), 5 × 36 singles (1×, 2×, 3×, 4×, 5× and 6× dose), 5 × 57 multidrug mixes); 1–5 timepoints; 2 time ranges (short-term and long-term killing), at least 2 independent replicates and 4 to 8 platings (dilutions + technical replicates).
Automated image analysis
The number of red and green colonies in each microplating spot was counted using a custom Python script64, implementing the following steps. (1) The GFP and DsRed microplating images (one for each microplating spot) were contrast-enhanced and noise-reduced (mean shift pyramid). (2) These enhanced GFP and DsRed images were thresholded (mahotas.otsu), yielding two corresponding binary images. (3) An exact Euclidean distance transform was used to yield a distance matrix of each pixel to its nearest zero pixel (scipy.ndimage.distance_transform_edt). (4) Local peaks in the distance matrix (skimage.feature.peak_local_max) were clustered into connected components (nearest neighbours, scipy.ndimage.label) to yield labelled feature arrays. (5) These feature arrays were used as seeds to the watershed algorithm to segregate the colonies (skimage.morphology.watershed). (6) Thresholding on the basis of the size and circularity of the colonies was applied to remove very tiny colonies or merged spots. (7) Microplating spots where the colonies merged extensively or with colonies that were too dense to reliably count were designated uncountable (over 100 colonies per spot). (8) For each given culture and time point, the colony counts at dilutions at which there was no uncountable plating were used to evaluate the CFU per ml (total number of colonies in all of the countable dilutions divided by total culture volume plated). Our high-throughput viability assay was highly reproducible, showing good agreement among biological replicates (Extended Data Fig. 10e–h). Co-cultures of GFP/DsRed strains enabled us to: (1) increase the sensitivity of the colony-detection algorithm—different colour colonies can be more easily distinguished even if they grow at close proximity; (2) spot low-frequency antibiotic-resistant mutants that manifest as red-only or green-only colonies on the agar spot where two identical strains labelled with different fluorescent tags; (3) conduct competition experiments in which the sensitive and resistant strains were labelled differentially (see below).
Calculating early-killing and clearance interaction scores between drug pairs
Early-killing and long-term clearance directional interaction scores (DIS) among pairwise combinations of the 14 antibiotics were quantified based on the fraction of surviving cells S after short (average of the 1 and 1.5 h time points; the killing delay was almost always shorter than half an hour across the different conditions indicating that the average cell survival after 1 and 1.5 h is an effective killing score that combines both the killing rate and drug action delay; Extended Data Fig. 10i) and long (8 h time point; the choice of 8 h incubation time is often used25,26,65,66, although note that longer times are used in some of the studies13,67,68) antibiotic exposures, respectively. For each drug A of the 14 antibiotics, a fixed cidal concentration (A1×) was chosen on the plateau phase of the dose–response curves (Extended Data Fig. 2a and Supplementary Table 1). For each drug pair A and B, cell survival under each drug alone at these chosen concentrations (SA(1×), SB(1×)), in double these concentrations (SA(2×), SB(2×)) and in a mix of the two drugs (SA(1×)+B(1×)) was evaluated. Then, the effect of drug B on drug A (DISB→A) and the effect of drug A on drug B (DISA→B) were defined on the basis of a comparison of the joint drug action SA(1×)+B(1×) with four reference idealized values. Specifically, the effect of drug B on A was defined as no-killing (SA(1×)+B(1×) = 1, DISB→A = 4), no effect (SA(1×)+B(1×) = S...
Comments
Post a Comment